Abstract
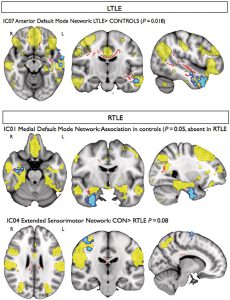
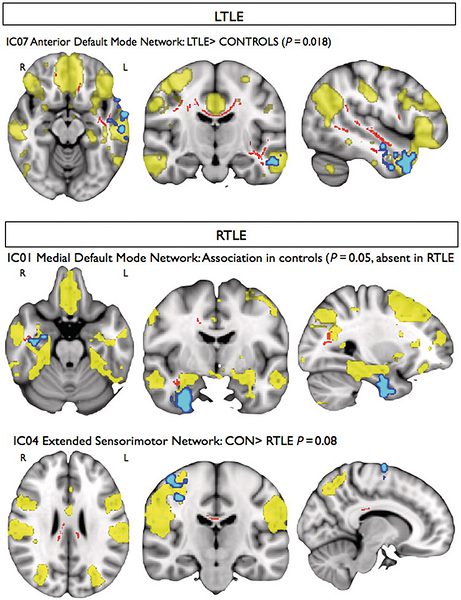
Magnetic resonance imaging methods that measure interregional brain signalling at rest have been advanced as powerful tools to probe organizational properties of functional networks. In drug-resistant temporal lobe epilepsy, resting functional magnetic resonance imaging studies have primarily employed region of interest approaches that preclude a comprehensive evaluation of large-scale functional interactions. In line with the distributed nature of structural damage in this condition, we set out to quantify connectivity across the entire range of resting networks. Furthermore, we assessed whether connectivity is driven by co-localized structural pathology. We obtained resting state, diffusion tensor and anatomical imaging data in 35 patients with temporal lobe epilepsy and 20 healthy subjects on a 3 T scanner. Resting state networks were identified using independent component analysis, which allows an objective whole-brain quantification of functional connectivity. We performed group comparisons before and after correcting for voxel-wise grey matter density. In addition, we identified voxel-wise associations between resting connectivity and white matter coherence indexed by fractional anisotropy. Compared with controls, patients showed altered (typically reduced) functional connectivity between the hippocampus, anterior temporal, precentral cortices and the default mode and sensorimotor networks. Reduced network integration of the hippocampus was explained by variations in grey matter density, while functional connectivity of the parahippocampus, and frontal and temporal neocortices showed atypical associations with white matter coherence within pathways carrying connections of these regions. Our multimodal imaging study suggests that in temporal lobe epilepsy, cortical atrophy and microstructural white matter damage impact functional resting connectivity.